Complex electronic interplay of σ-hole and π-hole interactions in crystals of halogen substituted 1,3,4-oxadiazol-2(3H)-thiones†
Abstract
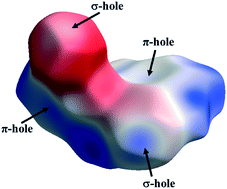
In this study, we have performed a detailed analysis of the nature and strength of different intermolecular interactions present in the crystal structures of four biologically active derivatives of 1,3,4-oxadiazol-2(3H)-thiones. The study was primarily focused on obtaining detailed physical insights into the differential nature of the σ-hole and π-hole interactions that exist in the solid state in this class of compounds. It is found that the directionality of an interaction plays a very important role in categorizing these interactions as being σ-hole and π-hole in origin. The presence of a σ-hole (on the S, Cl and Br atoms) and π-holes (in the oxadiazole and benzyl rings) is clearly evident from the molecular electrostatic potential maps. The presence of these ubiquitous interactions was further confirmed via QTAIM analysis by the presence of (3, −1) bond critical points (bcps) between the interacting atoms with acceptable topological parameters (ρbcp; ∇2ρbcp). Furthermore, Hirshfeld 2D fingerprint plots helped in quantitatively determining the role of different interactions in the crystal packing of the four reported structures.
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...