Growth of well-developed LaOCl microplates by chloride salt-assisted method†
Abstract
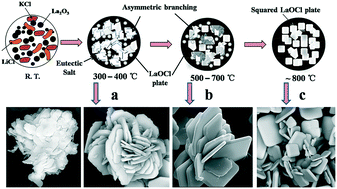
Lanthanum oxychloride (LaOCl) microplates were synthesized via the chloride salt-assisted method using lanthanum oxide, potassium chloride and lithium chloride as the raw materials. The effects of salt type and reaction temperature on the synthesis of LaOCl were investigated in detail. The lowest synthesis temperature in the present study was 300 °C, which was much lower than that required by other methods. The thickness and width of the as-prepared LaOCl microplates were about 200–500 nm and several microns, respectively. With potassium chloride and lithium chloride as the reaction medium, the latter acted not only as the molten salt but also as the reactant in the LaOCl synthesis. The chloride salt not only significantly lowers the synthesis temperature, but also provides the good conditions for the LaOCl growth of plate-like structures.


 Please wait while we load your content...
Please wait while we load your content...