From nanoparticles to bulk crystalline solid: nucleation, growth kinetics and crystallisation of mixed oxide ZrxTi1−xO2 nanoparticles
Abstract
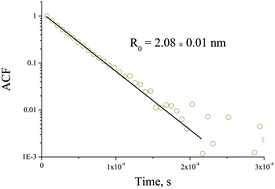
We describe the preparation of mixed metal oxide nanoparticles of a desirable composition and their transformation to the crystalline solids ZrxTi1−xO2 (0.0 ≤ x ≤ 1.0) after heat treatment. The correlation analysis between the size of the nucleus, the crystalline phase and the elemental composition of the solid is presented. Mixed metal oxide zirconium–titanium-oxo-alkoxy (ZTOA) nanoparticles of different elemental compositions 0 ≤ x = CZr/(CZr + CTi) ≤ 1 were prepared via the sol–gel method in a reactor by rapid micromixing of n-propanol fluids containing the precursors and water. The structural transformation of the nanoparticles takes place in two temperature ranges, 210–250 °C and 380–680 °C, which sensitively depends on the elemental composition. In the range 0.3 ≤ x ≤ 0.6, stable ZTOA nanoparticles with a radius of 2.1 ± 0.05 nm appeared at the hydrolysis ratio H ≤ 1.5. The heat treatment results in a single orthorhombic ZrxTi1−xO2 phase. The crystallisation onset temperature was the highest in this range of x, attaining 680 °C at x = 0.5. In the range 0 ≤ x ≤ 0.2, the particle radius decreased to 1.6 nm for pure titanium-oxo-alkoxy nuclei (TOA, x = 0); their heat treatment resulted in a single TiO2 anatase phase. In the range 0.7 ≤ x ≤ 1, the particle radius decreased to 1.8 nm for pure zirconium-oxo-alkoxy nuclei (ZOA, x = 1); their heat treatment resulted in mixed monoclinic and tetragonal ZrO2 phases. The crystalline cell parameters of the observed phases underwent a continuous variation with x. TEM images evidenced the nanoporous structure of submicronic orthorhombic ZrxTi1−xO2 monocrystals with a mean pore size of about that of the ZTOA nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...