Abstract
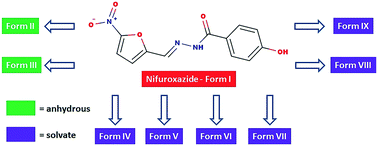
This paper describes the rational search for and the synthesis of eight new polymorphs, solvates or hydrates of the well-known drug nifuroxazide. By selecting the appropriate polymorph, a 50% increase in solubility was obtained, compared to the commercially available solid form. The target molecule was chosen using hydrogen bond propensity models as a rational approach. All new forms were characterized by X-ray powder diffraction and thermal methods, and, in three cases, by single crystal X-ray diffraction. Two new solid anhydrous polymorphs were discovered and showed a significant increase in solubility and dissolution rates in water over the known solid form.


 Please wait while we load your content...
Please wait while we load your content...