The formation of two thiotriazoline polymorphs: study from the energetic viewpoint†
Abstract
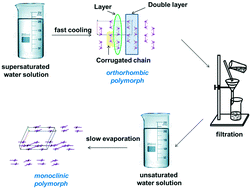
A comprehensive study of two polymorphic modifications of morpholin-4-ium 2-(5-methyl-1H-1,2,4-triazol-3-ylsulfanyl)acetate (trade mark “thiotriazoline”) within an approach based on the comparison of pairwise interaction energies has revealed the dependence of the crystal structure on the crystallization conditions. The orthorhombic modification is formed under kinetic control and has a more isotropic topology of intermolecular interaction energies. The building unit of the orthorhombic structure is a whole organic salt. The thermodynamically controlled crystallization causes the formation of the monoclinic modification with an anisotropic topology of interaction energies and the cation–anion pair dimer as a building unit. The main difference of the intermolecular interactions set within the two polymorphic modifications is associated with the presence or absence of non-charge-assisted hydrogen bonds with small enough energy.


 Please wait while we load your content...
Please wait while we load your content...