Methyl substituent effect on structure, luminescence and semiconducting properties of furan/phenylene co-oligomer single crystals†
Abstract
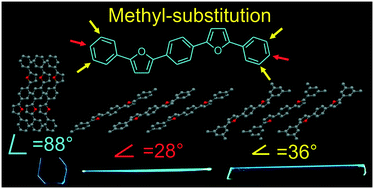
Single crystals of furan/phenylene co-oligomers are among the most promising highly-emissive materials for applications in various optoelectronic devices. In this work, we synthesized and studied furan/phenylene co-oligomers with the same conjugated core 1,4-bis(5-phenylfuran-2-yl)benzene and methyl substituents at p- and m-positions of the terminal phenyls. The effect of substituents on the crystal packing, charge transport and luminescence of the single crystals was studied. Compared to the unsubstituted compound, the methyl-substituted co-oligomers demonstrated improved thermostability and enhanced photoluminescence, which we assign to J-aggregation resulting from the strong inclination of the molecules against the main crystal facet. The charge mobility in single crystal organic field-effect transistors decreased upon the inclination of the molecules. We conclude that the molecular tilt angle, intermolecular distances and interactions in crystals of heteroaryl-containing linear conjugated oligomers can be controlled by the introduction of end methyl groups in the appropriate positions.


 Please wait while we load your content...
Please wait while we load your content...