Five different pseudo-polymorphs of 4-aminoarylphosphate: supramolecular aggregation in organophosphates†
Abstract
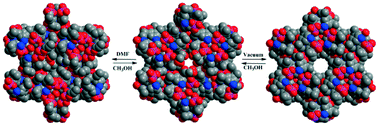
2,6-Diisopropyl-4-nitrophenylphosphate (A), synthesized in two steps from 2,6-diisopropyl-4-nitrophenol, serves as a convenient starting point to prepare several N-functionalized monoaryl phosphates via 2,6-diisopropyl-4-aminophenylphosphate (adippH2) (1), which quite interestingly exhibits five different pseudo-polymorphic structures 1a–1e. Several of these five polymorphic forms are inter-convertible to one another through single crystal to single crystal (SC–SC) transformation, aided by evacuation and solvent soaking protocols. Crystallization of crude 1 in methanol results in the isolation of the first polymorph [adippH2·CH3OH·1/3H2O]3 (1a), while evacuation of 1a results in the loss of solvent molecules from the crystal lattice to yield a new polymorph [adippH2·1/3H2O]3 (1b). Polymorph 1b can be converted back to 1a by soaking the single crystals of 1b in methanol. Similarly, soaking single crystals of 1a in DMF or in water yields two more new polymorphs [adippH2·1/3CH3OH·1/3DMF·1/3H2O]3 (1c) and [adippH2·1/2H2O]2 (1d), respectively. Polymorph 1c can once again be re-transformed to the original 1a by soaking its single crystals in methanol, whereas the SC–SC conversion of 1a to 1d is found to be irreversible. Polymorph 1e is obtained by crystallization of 1a in dry methanol. Interaction of adippH21 with triflic acid and 1,10-phenanthroline yields the corresponding salts 2 and 3, respectively. Acetylation of 1a results in the isolation of acetylamino derivative 4. Phosphate diesters 5 and 6 featuring both aryloxy and alkoxy substituents on the central phosphorus have also been synthesized by a sequential reaction starting from (4-NO2-2,6-iPr2C6H2O)P(O)Cl2. All new compounds have been exhaustively characterized by analytical and spectroscopic methods apart from establishing their crystal structures by single crystal X-ray diffraction studies. Owing to the simultaneous presence of phosphate ester groups (featuring P–OH and P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities), para amino or nitro group on the aryl substituent, and solvent molecules such as methanol and water, the crystal structures of 1a–1e and 2–6 exhibit interesting supramolecular aggregation that is primarily aided by H-bond networks.
O functionalities), para amino or nitro group on the aryl substituent, and solvent molecules such as methanol and water, the crystal structures of 1a–1e and 2–6 exhibit interesting supramolecular aggregation that is primarily aided by H-bond networks.


 Please wait while we load your content...
Please wait while we load your content...