Structural evolution of a metal–organic framework and derived hybrids composed of metallic cobalt and copper encapsulated in nitrogen-doped porous carbon cubes with high catalytic performance†
Abstract
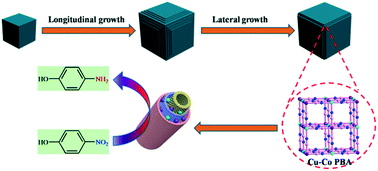
We present here the structural evolution of a Prussian blue analogue CuII3[CoIII(CN)6]2 (Cu–Co PBA) controlled by sodium citrate as a function of time at room temperature. We identified the different stages of Cu–Co PBA formation and elucidated its transformation kinetics. Moreover, 3D hybrid composites of nitrogen-doped porous carbon cubes, with cobalt and copper nanoparticles embedded within the well-graphitized shells, were rationally fabricated using Cu–Co PBA as the single precursor. The resulting Cu/Co@NPCC catalysts showed superior catalytic activity in the reduction of 4-nitrophenol to 4-aminophenol. Metallic cobalt and copper cores were leached out by FeCl3 and HCl treatment to determine the catalytically active sites. Results showed that the nitrogen-doped porous carbon still exhibited excellent catalytic performance after the metallic cores were removed. Based on the experimental results, we speculate that the unique structure of metal residues encapsulated inside the graphitic carbon layers may have higher catalytic activity than the metal particles themselves.

 Please wait while we load your content...
Please wait while we load your content...