Palladium-catalyzed enantioselective C(sp2)–H arylation of ferrocenyl ketones enabled by a chiral transient directing group†
Abstract
Palladium-catalyzed enantioselective C(sp2)–H activation of ferrocenyl ketones is achieved through utilizing catalytic, inexpensive L-tert-leucine as a chiral transient directing group. The transformation allows rapid access to ferrocene scaffolds simultaneously possessing planar- and stereogenic central chirality, widely applied in the ferrocene-based chiral ligand families.
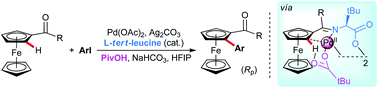


 Please wait while we load your content...
Please wait while we load your content...