Dissociation of haemolytic and oligomer-preventing activities of gramicidin S derivatives targeting the amyloid-β N-terminus†
Abstract
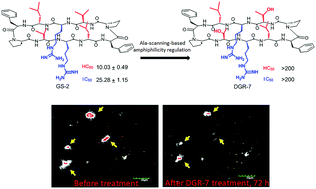
The intrinsic haemolysis of an amyloid-β (Aβ) N-terminal targeting gramicidin S derivative was successfully dissociated from its Aβ oligomer-preventing activities via Ala-scanning-based regulation of molecular amphiphilicity. The representative analogue DGR-7 shows low toxicity but significant efficiency in preventing Aβ oligomers and reducing amyloid plaques in APP/PS1 transgenic AD mice.


 Please wait while we load your content...
Please wait while we load your content...