Electrochemical hydrogenation of a benzannulated pyridine to a dihydropyridine in acidic solution†
Abstract
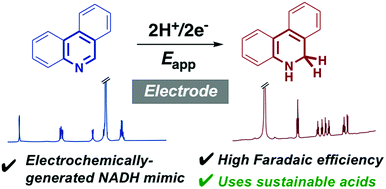
The electrochemistry of pyridines in acidic solution is dominated by a ‘weak acid’ reduction on the cyclic voltammetry timescale. Here we show that electrochemical hydrogenation of a benzannulated pyridine, phenanthridine (1), to the biomimetic hydride donor 1,2-dihydrophenanthridine (1-H2) can occur selectively at glassy carbon electrodes over longer timescales of potentiostatic electrolysis.


 Please wait while we load your content...
Please wait while we load your content...