Ligand-induced reactivity of β-diketiminate magnesium complexes for regioselective functionalization of fluoroarenes via C–H or C–F bond activations†
Abstract
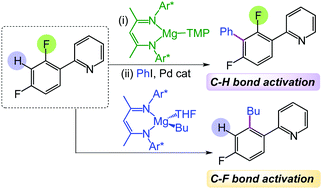
Using β-diketiminate Mg(II) complexes containing either alkyl, aryl or amide groups, the regioselective functionalization of a wide range of fluoroarenes is accomplished but in uniquely different ways. Overcoming common limitations of traditional s-block bases, kinetically activated [(DippNacnac)Mg(TMP)] (1) deprotonates these molecules at room temperature, trapping sensitive fluoroaryl anions that can then engage in Negishi cross-coupling; whereas [(DippNacnac)Mg(R)THF] (R = nBu, Ph, benzofuryl) have proved to be effective reagents for C–F bond alkylation/arylation via pyridine directed C–F bond cleavage.


 Please wait while we load your content...
Please wait while we load your content...