Regiocontrolled direct C4 and C2-methyl thiolation of indoles under rhodium-catalyzed mild conditions†
Abstract
A straightforward Rh(III)-catalyzed general strategy was developed for the site-selective remote C4 (sp2) and C2 (sp3)-methyl thiolation of an indole core, keeping the oxime directing group at the C3 position. The transformation was accomplished under mild conditions with a wide scope and functional group tolerance. The directing group can easily be removed after operation. Methyl substitution at the C2 position of the indole core led to C2 (sp3)-methyl thiolation.
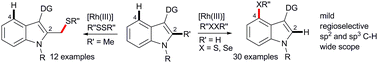


 Please wait while we load your content...
Please wait while we load your content...