An exploding N-isocyanide reagent formally composed of anthracene, dinitrogen and a carbon atom†
Abstract
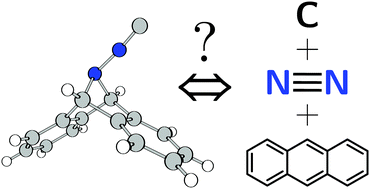
Targeted as an example of a compound composed of a carbon atom together with two stable neutral leaving groups, 7-isocyano-7-azadibenzonorbornadiene, CN2A (1, A = C14H10 or anthracene) has been synthesized and spectroscopically and structurally characterized. The terminal C atom of 1 can be transferred: mesityl nitrile oxide reacts with 1 to produce carbon monoxide, likely via intermediacy of the N-isocyanate OCN2A. Reaction of 1 with [RuCl2(CO)(PCy3)2] leads to [RuCl2(CO)(1)(PCy3)2] which decomposes unselectively: in the product mixture, the carbide complex [RuCl2(C)(PCy3)2] was detected. Upon heating in the solid state or in solution, 1 decomposes to A, N2 and cyanogen (C2N2) as substantiated using molecular beam mass spectrometry, IR and NMR spectroscopy techniques.


 Please wait while we load your content...
Please wait while we load your content...