Aza-capped cyclodextrins for intra-cavity metal complexation†
Abstract
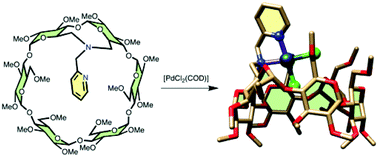
Aza-capped, methylated cyclodextrins (CDs) were obtained in high yields by reacting the soft nitrogen nucleophile 2-nitrobenzenesulfonamide with either A,B-dimesylated CDs in basic media or their diol analogues under Mitsunobu reaction conditions followed by deprotection with thiophenol. A methyl pyridine substituent was grafted on the N atom of these secondary amines. When built on an α-CD scaffold, the resulting tertiary amine no longer undergoes nitrogen inversion at room temperature and behaves as a confining ligand, opening the way to intra-cavity metal complexation and promoting the formation of supramolecular helices.


 Please wait while we load your content...
Please wait while we load your content...