Cyclohexa-1,3-diene-based dihydrogen and hydrosilane surrogates in B(C6F5)3-catalysed transfer processes†
Abstract
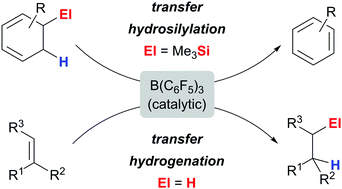
The cyclohexa-1,3-diene motif is introduced as an equally effective alternative to the cyclohexa-1,4-diene platform in B(C6F5)3-catalysed transfer processes. The transfer hydrogenation of alkenes is realised with α-terpinene and the related transfer hydrosilylation is achieved with 5-trimethylsilyl-substituted cyclohexa-1,3-diene. Both yields and substrate scope are comparable with the prior systems.


 Please wait while we load your content...
Please wait while we load your content...