Prebiotic synthesis of 2-deoxy-d-ribose from interstellar building blocks promoted by amino esters or amino nitriles†
Abstract
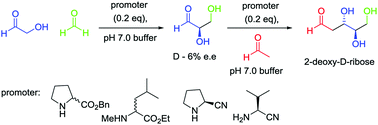
Understanding the prebiotic genesis of 2-deoxy-D-ribose, which forms the backbone of DNA, is of crucial importance to unravelling the origins of life, yet remains open to debate. Here we demonstrate that 20 mol% of proteinogenic amino esters promote the selective formation of 2-deoxy-D-ribose over 2-deoxy-D-threopentose in combined yields of ≥4%. We also demonstrate the first aldol reaction promoted by prebiotically-relevant proteinogenic amino nitriles (20 mol%) for the enantioselective synthesis of D-glyceraldehyde with 6% ee, and its subsequent conversion into 2-deoxy-D-ribose in yields of ≥ 5%. Finally, we explore the combination of these two steps in a one-pot process using 20 mol% of an amino ester or amino nitrile promoter. It is hence demonstrated that three interstellar starting materials, when mixed together with an appropriate promoter, can directly lead to the formation of a mixture of higher carbohydrates, including 2-deoxy-D-ribose.


 Please wait while we load your content...
Please wait while we load your content...