A stereoselective thiocyanate conjugate addition to electron deficient alkynes and concomitant cyclization to N,S-heterocycles†
Abstract
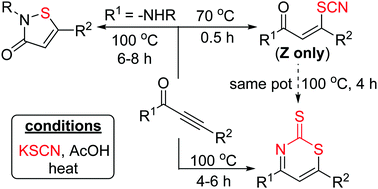
A regio- and stereoselective thiocyanate addition to ynones is achieved using KSCN in AcOH at 70 °C. The reaction is extendable to ynals, ynesulfones, ynoic acids and ynoates. Adducts from ynones were readily transformed into thiazine-2-thione derivatives under slightly modified reaction conditions. In contrast, thiocyanated adducts from ynamides underwent an in situ decyanative amido cyclization towards isothiazolones. None of these events needed any transition metal or catalyst, attaining a high synthetic value.


 Please wait while we load your content...
Please wait while we load your content...