Cyclic peptide production using a macrocyclase with enhanced substrate promiscuity and relaxed recognition determinants†
Abstract
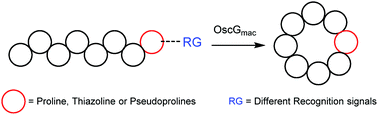
Macrocyclic peptides have promising therapeutic potential but the scaling up of their chemical synthesis is challenging. The cyanobactin macrocyclase PatGmac is an efficient tool for production but is limited to substrates containing 6–11 amino acids and at least one thiazoline or proline. Here we report a new cyanobactin macrocyclase that can cyclize longer peptide substrates and those not containing proline/thiazoline and thus allows exploring a wider chemical diversity.


 Please wait while we load your content...
Please wait while we load your content...