A covalent G-site inhibitor for glutathione S-transferase Pi (GSTP1-1)†
Abstract
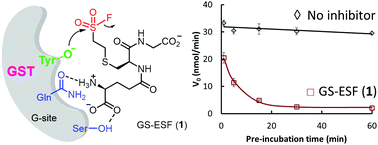
We herein report the first covalent G-site-binding inhibitor for GST, GS-ESF (1), which irreversibly inhibited the GSTP1-1 function. LC-MS/MS and X-ray structure analyses of the covalently linked GST-inhibitor complex suggested that 1 reacted with Tyr108 of GSTP1-1. The mechanism of covalent bond formation was discussed based on MD simulation results.


 Please wait while we load your content...
Please wait while we load your content...