Halogen-bonded iodonium ion catalysis: a route to α-hydroxy ketones via domino oxidations of secondary alcohols and aliphatic C–H bonds with high selectivity and control†
Abstract
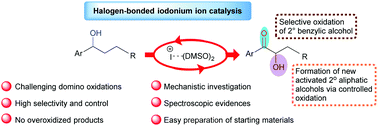
A domino synthesis of α-hydroxy ketones has been developed from benzylic secondary alcohols employing catalytic iodonium ions stabilized by DMSO. The reaction proceeds through an unprecedented sequential oxidation of alcohols to ketone and its α-hydroxylation in a controlled manner. The spectroscopic evidence establishes the possibility of formation of a stable halogen-bonded adduct between DMSO and iodonium ions.


 Please wait while we load your content...
Please wait while we load your content...