Direct, efficient NHC-catalysed aldehyde oxidative amidation: in situ formed benzils as unconventional acylating agents†
Abstract
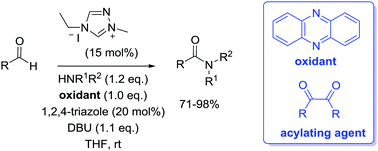
A new N-heterocyclic carbene-catalysed oxidative amidation of aldehydes has been developed which converts the aldehyde to a benzil acylating agent in situ. The process uses an air-recyclable oxidant and a nucleophilic co-catalyst and does not require the use of a large excess of either one coupling partner or catalyst.


 Please wait while we load your content...
Please wait while we load your content...