Synthetic route to chiral indolines via Cu(OAc)2-catalyzed ring-opening/C(sp2)–H activation of activated aziridines†
Abstract
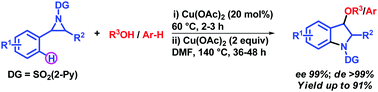
A simple strategy for the synthesis of highly functionalized indolines via Lewis acid catalyzed ring-opening of activated aziridines with various nucleophiles followed by Cu(OAc)2-mediated intramolecular C–H amination in one-pot has been developed with excellent enantio- and diastereospecificity (ee 99%; de >99%). The reaction proceeds via Cu(OAc)2-catalyzed SN2-type ring-opening of 2-phenyl-N-(2-pyridinesulfonyl)aziridine with alcohols and arene, followed by copper-mediated pyridine-2-sulfonamide directed intramolecular C(sp2)–H activation/cyclization in a stepwise fashion to furnish the indoline derivatives in excellent yields (up to 91%).


 Please wait while we load your content...
Please wait while we load your content...