A one-pot tandem chemoselective allylation/cross-coupling via temperature control of a multi-nucleophile/electrophile system†
Abstract
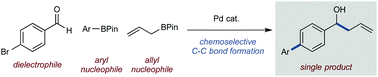
A chemoselective tandem reaction of a multi-reactive, two electrophile + two nucleophile, system is reported. An allylation/cross-coupling process of a haloaryl aldehyde, an aryl BPin, and an allyl BPin can be controlled using a temperature gradient to overcome natural reactivity profiles and allow two sequential chemoselective C–C bond formations without intervention. This process offers efficient access to an array of functionalised products including pharmaceutical and natural product scaffolds.
- This article is part of the themed collection: Celebrating our 2019 Prize and Award winners


 Please wait while we load your content...
Please wait while we load your content...