Nucleophilic trifluoromethylation of electron-deficient arenes†
Abstract
A novel trifluoromethylation of arenes is presented, which proceeds under mild reaction conditions and has the potential for late-stage functionalisation of pharmaceuticals and agrochemicals. The new reaction allows for the regioselective conversion of nitroarenes into 1,2-trifluoromethylated nitroarenes, via a C–H activation pathway. Furthermore, a substitution of nitroarenes to trifluoromethyl arenes is also presented.
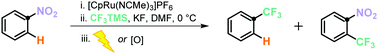


 Please wait while we load your content...
Please wait while we load your content...