Asymmetric alkylation of remote C(sp3)–H bonds by combining proton-coupled electron transfer with chiral Lewis acid catalysis†
Abstract
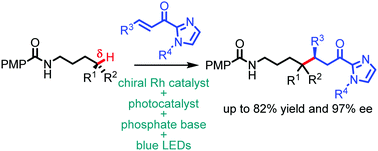
The catalytic asymmetric alkylation of the remote, unactivated δ-position of N-alkyl amides was enabled by the combination of visible-light-induced proton-coupled electron transfer, 1,5-hydrogen atom transfer, and chiral Lewis acid catalysis in up to 82% yield and up to 97% ee.


 Please wait while we load your content...
Please wait while we load your content...