Visible light sensitization of benzoyl azides: cascade cyclization toward oxindoles via a non-nitrene pathway†
Abstract
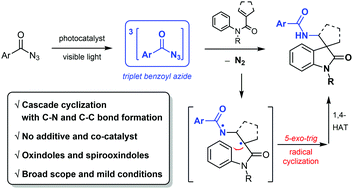
Visible light sensitization of benzoyl azides was examined in reaction with N-phenylmethacrylamides to afford biologically important oxindoles and spirooxindoles via a cascade cyclization under mild reaction conditions. Mechanistic studies suggested a non-nitrene pathway, where triplet benzoyl azides act as the reactive intermediate.


 Please wait while we load your content...
Please wait while we load your content...