A halogen bond route to shorten the ultrashort sextuple bonds in Cr2 and Mo2†
Abstract
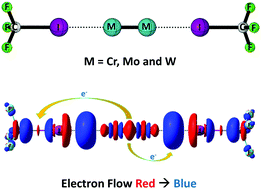
Sextuple bonded group 6 diatomics Cr2 and Mo2 possess ultrashort metal–metal bonds. Yet their bond dissociation energy is very low. The destabilising nature of σ-bonds is responsible for this. Selective extraction of these σ-electrons via a σ-hole on a halogen bond donor shortens and strengthens the metal–metal bond. This study constitutes a hitherto unexplored application of halogen bonding and an example for the true violation of bond order–bond strength relation.

 Please wait while we load your content...
Please wait while we load your content...