Bioelectrocatalytic NAD+/NADH inter-conversion: transformation of an enzymatic fuel cell into an enzymatic redox flow battery†
Abstract
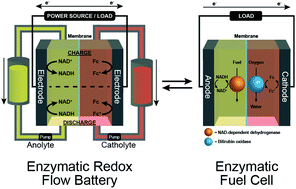
Diaphorase and a benzylpropylviologen redox polymer were combined to create a bioelectrode that can both oxidize NADH and reduce NAD+. We demonstrate how bioelectrocatalytic NAD+/NADH inter-conversion can transform a glucose/O2 enzymatic fuel cell (EFC) with an open circuit potential (OCP) of 1.1 V into an enzymatic redox flow battery (ERFB), which can be rapidly recharged by operation as an EFC.


 Please wait while we load your content...
Please wait while we load your content...