Efficient conversion of N6-threonylcarbamoyladenosine (t6A) into a tRNA native hydantoin cyclic form (ct6A) performed at nucleoside and oligoribonucleotide levels†
Abstract
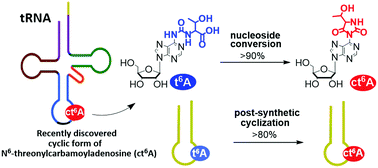
A t6A nucleoside was efficiently and stereospecifically transformed into a hydantoin cyclic form of N6-L-threonylcarbamoyladenosine (ct6A) by the use of polymer bounded carbodiimide (EDC-P) and HOBt. The procedure was successfully applied for a post-synthetic conversion of t6A-containing RNA 17-mers (of the sequences of anticodon stem and loop (ASL) fragments of S. pombe tRNAi and E. coli tRNALys) into the products bearing the ct6A unit.


 Please wait while we load your content...
Please wait while we load your content...