A thermodynamic and kinetic analysis of solvent-enhanced selectivity in monophasic and biphasic reactor systems†
Abstract
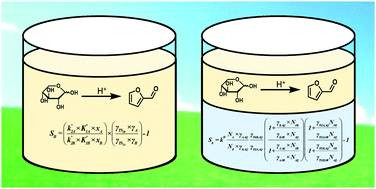
Selectivity control in biomass conversion is often realized by manipulating the solvent environment. Outcomes can be rationalized through a thermodynamically rigorous application of transition-state theory. We show that solvent-induced perturbations to selectivity in both monophasic and biphasic reactor systems are governed by the same underlying principles.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...