Orthogonal electronic coupling in multicentre arylamine mixed-valence compounds based on a dibenzofulvene–thiophene conjugated bridge†
Abstract
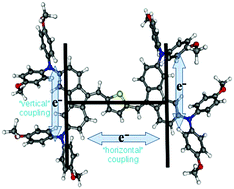
Herein we present organic mixed-valence compounds with an innovative H-shape design, where four redox centres are bridged “vertically” via a dibenzofulvene backbone and “horizontally” via a bis-(dibenzofulvene)–thiophene bridge. These compounds are easily oxidized to stable highly charged radical species which show intense intervalence charge transfer transitions in the near infrared region. Interestingly, depending on the position of the arylamine substituents on the bridge, both vertical and horizontal electron transfer pathways can be optically induced.


 Please wait while we load your content...
Please wait while we load your content...