Size-dependent conformational change in halogen–π interaction: from benzene to graphene†
Abstract
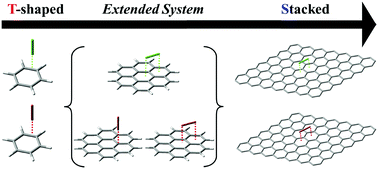
Diatomic halogen molecules X2 (X = Cl/Br) favor the edge-to-face conformation on benzene with significant electrostatic interaction via halogen bonding. In contrast, they favor the stacked conformation on graphene with negligible electrostatic interaction. As the aromatic ring expands, the inner facial side becomes almost electrostatically neutral. On coronene, the two conformations are compatible.


 Please wait while we load your content...
Please wait while we load your content...