Fast Mg2+ diffusion in Mo3(PO4)3O for Mg batteries†
Abstract
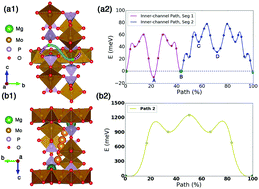
In this work, we identify a new potential Mg battery cathode structure Mo3(PO4)3O, which is predicted to exhibit ultra-fast Mg2+ diffusion and relatively high voltage based on first-principles density functional theory calculations. Nudged elastic band calculations reveal that the migration barrier of the percolation channel is only ∼80 meV, which is remarkably low, and comparable to the best Li-ion conductors. This low barrier is verified by ab initio molecular dynamics and kinetic Monte Carlo simulations. The voltage and specific energy are predicted to be ∼1.98 V and ∼173 W h kg−1, respectively. If confirmed by experiments, this material would have the highest known Mg mobility among inorganic compounds.


 Please wait while we load your content...
Please wait while we load your content...