Asymmetric fluorinative dearomatization of tryptamine derivatives†
Abstract
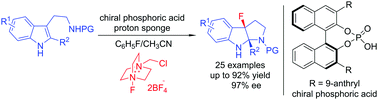
An asymmetric fluorinative dearomatization reaction of tryptamine derivatives was developed by using a chiral anion phase transfer catalyst (PTC) system, and the preliminary results of the reaction mechanistic study were achieved. This method is characterized by a simple operation, facile introduction of a fluorine atom in a highly enantioselective manner and construction of two contiguous quaternary stereogenic centers.


 Please wait while we load your content...
Please wait while we load your content...