Chemoselective ratiometric imaging of protein S-sulfenylation†
Abstract
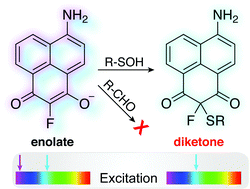
Here we report a ratiometric fluorescent probe for chemoselective conjugation to sulfenic acids in living cells. Our approach couples an α-fluoro-substituted dimedone to an aminonaphthalene fluorophore (F-DiNap), which upon sulfenic acid conjugation is locked as the 1,3-diketone, changing the fluorophore excitation. F-DiNap reacts with S-sulfenylated proteins at equivalent rates to current probes, but the α-fluorine substitution blocks side-reactions with biological aldehydes.
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...