Dissecting mechanism of coupled folding and binding of an intrinsically disordered protein by chemical synthesis of conformationally constrained analogues†
Abstract
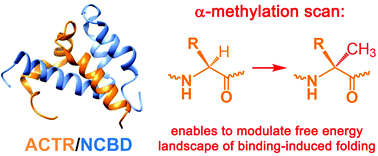
Non-canonical α-methyl amino acids were incorporated at various sites in the sequence of intrinsically disordered activation domain from the p160 transcriptional co-activator (ACTR) to facilitate the formation of α-helical structures. Kinetic and thermodynamic data confirm the induced fit mechanism of complex formation between the synthesized ACTR variants and the nuclear co-activator binding domain (NCBD).
- This article is part of the themed collection: 2017 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...