Copper-catalyzed asymmetric alkynylation of cyclic N-sulfonyl ketimines†
Abstract
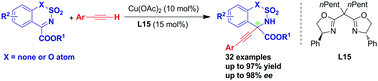
A Cu-catalyzed asymmetric alkynylation of cyclic N-sulfonyl ketimines was developed, providing the corresponding chiral α-tertiary amines with up to 98% ee. The method tolerates some variations in cyclic N-sulfonyl ketimine and alkyne scope. These products could be used in several transformations, in particular, the products of 6-membered cyclic N-sulfonyl ketimines could be easily converted to linear chiral α-tertiary amines. This asymmetric alkynylation provides an efficient, gram-scale, low-cost transition-metal catalyzed synthesis of chiral α-tetrasubstituted propargylamines.


 Please wait while we load your content...
Please wait while we load your content...