Iridium-catalysed asymmetric allylic alkylation of benzofuran γ-lactones followed by heteroaromatic Cope rearrangement: study of an unusual reaction sequence†
Abstract
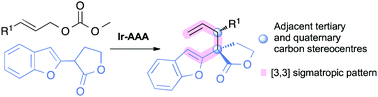
The iridium-catalysed asymmetric allylic alkylation of γ-lactones produces an all-carbon quaternary stereocentre substituted by an allyl and a benzofuran. The resulting 1,5-hexadienes were found to be excellent substrates for an unusual heteroaromatic Cope rearrangement. We describe here the first insight into the synthetic outcome of this intriguing reaction sequence.


 Please wait while we load your content...
Please wait while we load your content...