Effects of chalcogen atom substitution on the optoelectronic and charge-transport properties in picene-type π-systems†
Abstract
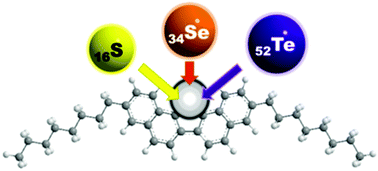
A series of π-conjugated 3,10-dialkyl-dinaphtho[1,2-b:2′,1′-d]chalcogenophenes incorporating S, Se, and Te as the central chalcogen atom was newly synthesized, and their optoelectronic and charge-transport properties were systematically investigated. High carrier mobilities of up to 4.7 cm2 V−1 s−1 were achieved for solution-processed organic field-effect transistors using these materials as p-channel organic semiconductors.


 Please wait while we load your content...
Please wait while we load your content...