Amino acid functionalisation using the 2-phosphaethynolate anion. A facile route to (phosphanyl)carbonyl-amino acids†
Abstract
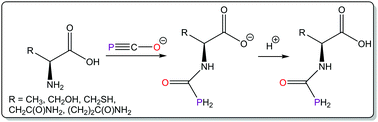
We describe the reactivity of the 2-phosphaethynolate anion (PCO−) towards enantiomerically pure α-amino acids (AAs) resulting in the formation of novel salts of phosphinecarboxamides bearing chiral functionalities. These transformations occurred quantitatively with all but one of the amino acids trialled (the basic amino acid arginine was found to be unreactive). The resulting ionic species can be readily protonated to afford N-(phosphanyl)carbonyl-amino acids, a novel group of amino acids bearing primary phosphine functionalities.
- This article is part of the themed collection: Celebrating our 2018 prize and award winners


 Please wait while we load your content...
Please wait while we load your content...