Palladium-catalyzed regioselective C–H fluoroalkylation of indoles at the C4-position†
Abstract
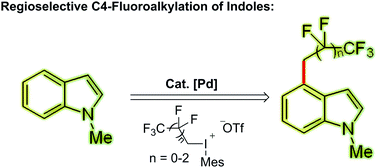
An exclusive catalytic C4-selective fluoroalkylation of indoles with highly active (1H, 1H-perfluoroalkyl)mesityliodonium triflate has been described. The key to its high regioselectivity is the appropriate choice of an easily accessible, cheap and removable directing group at the C3 position in the presence of a Pd(OAc)2 catalyst. Besides indole fluoroalkylation, the application of this strategy in other heteroarenes such as benzo[b]thiophene is also described.


 Please wait while we load your content...
Please wait while we load your content...