Utilization of electron-donating α,β-unsaturated oximes: regioselective inverse 1,3-dipolar cycloaddition of nitrones†
Abstract
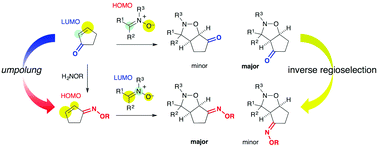
The cycloaddition of nitrones with α,β-unsaturated carbonyl compounds (enones) afforded predominantly 4-acylisoxazolidines, whereas the cycloaddition of the corresponding oximes afforded 5-iminoisoxazolidines. This inverse regioselection is due to HOMO activation by the oxime functionality.


 Please wait while we load your content...
Please wait while we load your content...