A radical coupled pathway to a stable and terminally bound titanium methylidene†
Abstract
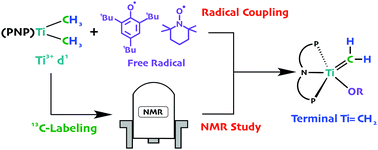
Radical coupling or oxidation of the titanium(III)dimethyl precursor (PNP)Ti(CH3)2 produced the dimethyl compounds, (PNP)Ti(CH3)2(X) (X = TEMPO, OMes*, OTf), which then thermally extrude methane to cyclometallate (X = TEMPO) or form the methylidene (X = OMes* or OTf). Various NMR spectral experiments in addition to 13C isotopic labeling, and structural studies are reported.


 Please wait while we load your content...
Please wait while we load your content...