Palladium-catalyzed interannular meta-C–H arylation†
Abstract
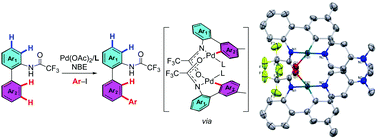
The interannular meta-selective C–H arylation of biaryl-2-trifluoroacetamides using Pd(II)/norbornene catalysis is reported. The installation of a trifluoroacetyl protecting group to tune their electronic properties and binding ability is essential for interannular selectivity. A dimeric palladacycle, comprising two cyclopalladated trifluoroacetamino biaryl units linked through trifluoroacetamide, was isolated and confirmed to be the key intermediate. Furthermore, the resulting products could be further elaborated via ipso-alkynylation and/or directed intraannular ortho-C–H functionalization, allowing access to various fully functionalized biaryl-2-amine derivatives.


 Please wait while we load your content...
Please wait while we load your content...