Regioselective magnesiation of N-heterocyclic molecules: securing insecure cyclic anions by a β-diketiminate-magnesium clamp†‡
Abstract
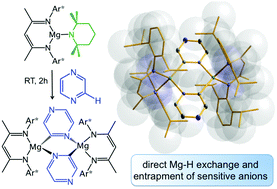
Using a specially designed magnesium metallating manifold, combining kinetically activated TMP amide base with a sterically amplified β-diketiminate ligand, this study has established a new regioselective strategy for magnesiation of challenging N-heterocyclic molecules. The broad scope of the approach is illustrated through reactions of pyrazine, triazoles and substituted pyridines by isolation and structural elucidation of their magnesiated intermediates.
- This article is part of the themed collection: Celebrating the 2017 RSC Prize and Award Winners


 Please wait while we load your content...
Please wait while we load your content...