Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H2S)†
Abstract
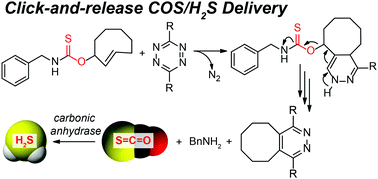
Hydrogen sulfide (H2S) is an important biomolecule with high therapeutic potential. Here we leverage the inverse-electron demand Diels–Alder (IEDDA) click reaction between a thiocarbamate-functionalized trans-cyclooctene and a tetrazine to deliver carbonyl sulfide (COS), which is quickly converted to H2S by the uniquitous enzyme carbonic anhydrase (CA), thus providing a new strategy for bio-orthogonal COS/H2S donation.
- This article is part of the themed collection: Most downloaded articles of 2017: Organic and Biological Chemistry


 Please wait while we load your content...
Please wait while we load your content...