Synthesis of chiral sultams via palladium-catalyzed intramolecular asymmetric reductive amination†
Abstract
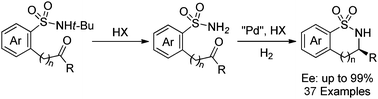
A novel palladium-catalyzed intramolecular reductive amination of ketones with weakly nucleophilic sulfonamides has been developed in the presence of a Brønsted acid, giving a wide range of chiral γ-, δ-, and ε-sultams in high yields and up to 99% of enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...