Defining the molecular structure of teixobactin analogues and understanding their role in antibacterial activities†
Abstract
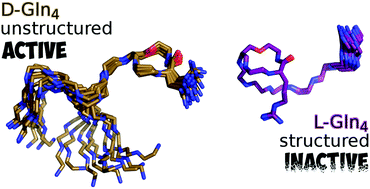
The discovery of the highly potent antibiotic teixobactin, which kills the bacteria without any detectable resistance, has stimulated interest in its structure–activity relationship. However, a molecular structure–activity relationship has not been established so far for teixobactin. Moreover, the importance of the individual amino acids in terms of their L/D configuration and their contribution to the molecular structure and biological activity are still unknown. For the first time, we have defined the molecular structure of seven teixobactin analogues through the variation of the D/L configuration of its key residues, namely N-Me-D-Phe, D-Gln, D-allo-Ile and D-Thr. Furthermore, we have established the role of the individual D amino acids and correlated this with the molecular structure and biological activity. Through extensive NMR and structural calculations, including molecular dynamics simulations, we have revealed the residues for maintaining a reasonably unstructured teixobactin which is imperative for biological activity.


 Please wait while we load your content...
Please wait while we load your content...