1,2-Difunctionalization-type (hetero)arylation of unactivated alkenes triggered by radical addition/remote (hetero)aryl migration†
Abstract
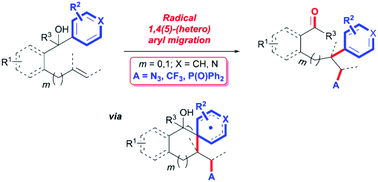
A novel difunctionalization-type (hetero)arylation of unactivated alkenes has been developed via remote 1,4(5)-(hetero)aryl migration triggered by radical alkene azidation, trifluoromethylation, or phosphonylation. The overall process serves as an unusual and reliable approach for straightforward access to diversely substituted ketones with broad functional group compatibility from readily available substrates and reagents.


 Please wait while we load your content...
Please wait while we load your content...